Cancer-associated fibroblasts (CAFs) in colon cancer development and progression
The risk of colon cancer is 20- to 30-fold higher in patients with ulcerative
colitis, than in a control population. These observations suggest the crucial
contribution of chronic inflammation to colon carcinogenesis.
Multiple colon tumors with nuclear accumulation of beta-catenin, develop
in mice after an intraperitoneal injection of a generator of O6-methylguanine, azoxymethane (AOM), followed by a repetitive oral intake
of dextran sodium sulfate (DSS) solution. Accumulating evidence indicate
the crucial role of the activation of a transcription factor NF-kappaB
in this model. We revealed that a potent NF-kappaB activator, TNF-alpha,
and a chemokine, CCL2, contribute to this colon carcinogenesis by inducing
the accumulation of cyclooxygenase (COX)-2 expressing leukocytes (Popivanova BK et al, 2008) (Popivanova BK et al., 2009).
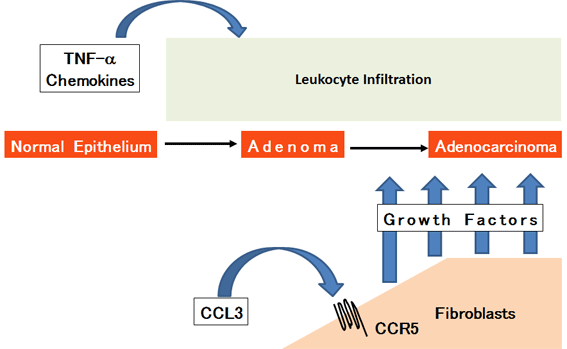
We further revealed that leukocyte infiltration is not sufficient for
colon cancer progression in this model and that fibroblast accumulation
is required for the progression of this colion cancer model. Moreover,
we demonstrated that colon tumor tissues produce CCL3, which attract fibroblasts
expressing CCR5, a specific receptor for CCL3. CCR5-expressing fibroblasts
contribute to colon cancer progression by providing cancer cells with a
myriad of growth factors. Similara mechanisms are also observed in colon
tumor tissues arising from subcutaneous or intracecal injection of syngeneic
colon adenocarcima cells into mice (Below Figure) (Sasaki et al., 2014).
We observed that a CCR5 antgonist, maraviroc, can decrease cancer-associated
fibroblasts (CAFs) and reduce tumor formation arising from intracecal injection
of either a mouse ora human colon adenocarcinoma cell line (Tanabe et al.,
2016).
Thus, the regulation of CAFs via CCR5 may be a novel therapeutic strategy
for colon cancer.
