Top of this page
Breast cancer bone metastasis
Bone metasis is complicated in most patients with breast cancer at advanced
stage and deteriorates markedly their life expectancy. Osteoclast activation
is presumed to be involved in bone metastasis and therfore, drugs targeting
osteoclast activation are used for bone metastasis but with limited efficacy.
Moreove, these drugs can cause severe adverse effects such as necrosis
of mandible and as a consequence, a novel theraputic strategy is required
to be developed based on the clarification of cellular and molecular mechanisms
of bone metastasis.
From a murine estrogen receptor-, progesterone receptor-, and Her2-negative
breasr cancer cell line, 4T1, we established 4T1.3 clone with a high capacity
to metastasize to bone upoin its injection into mammary fat pad, by repeating
the in vitro propagation of cancer cells present in bone after injection
into mammary fat pad.
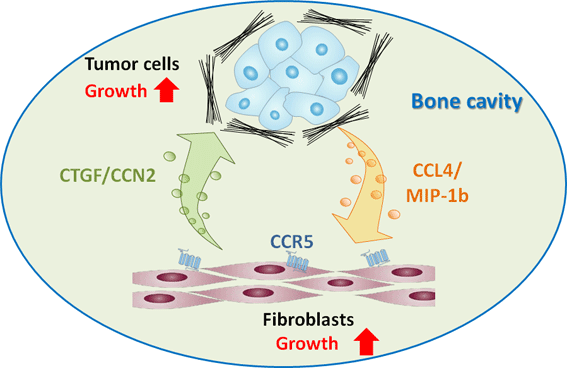
Compared with a parental clone, 4T1.3 clone did not show any differences in in vitro proliferation, tumor growth rates at the primary site, and extravasation. However, compared with a parental clone, 4T1.3 clone formed a larger tumor mass when injected into bone cavity. DNA microarray analysis under in vitro culture conditions, revealed that 4T1.3 clone exhibited enhanced expression of a chemokine, CCL4, without discernible expression of CCR5, a specific receptor for CCL4.
Intrabone injection of 4T1,3 increased type I collagen-positive cells in bone without few effects on osteoclasts and osteblasts, compared with that of a parental clone. Intrabone injection of CCL4 shRNA-treated 4T1.3 clone or intrabone injection of 4T1.3 clone into CCR5-deficient mice, abrogagted an increase in type I collagen-positive cell numbers and reduced tumor formation.
Intrabone injection of 4T1.3 enhanced the expression of connective tissue
growth factor (CYGF/CCN2). Moreover, CTGF/CCN2 expression was restricted
to CCR5-positive and type I collagen-positive cells. Finally, under in
vitro culture conditions、CCL4 induced fibroblasts to proliferate and to
express CTGF/CCN2 while CTGF/CCN2 augemented the cell proliferation of
4T1.3 clone under hypoxic conditions.
Collectively, there exist a positive feedback between breast cancer cells
and fibroblasts in bone, where breast cancer cells produce CCL4, which
acts onCCR5-expressin fibroblasts to produdce CTGF/CCN2, the factor with
an ability to promote breast cancer proliferation (Below Figure)(Sasaki et al., 2016).
Moreover, these observations raise a possibility to develop anti-bone metastasis
strategy by targeting fibroblasts in bone.