Organization
Division of Genetics
Staff

Professor
OSHIMA, Masanobu

Associate Professor
OSHIMA, Hiroko

Associate Professor
NAKAYAMA, Mizuho

Assistant Professor
WANG, Dong
Aims and Major projects
Accumulating evidence has indicated that genetic alterations as well as tumor microenvironment play a key role in cancer development and malignant progression. To examine the underlying mechanisms, we have constructed novel mouse and organoid models for gastrointestinal tumors and performed following projects.
【Cancer progression by Kras/p53 mutation and TGFβ】
Using mouse intestinal tumor-derived organoids, we found that organoids carrying Kras G12D and p53 R270H mutations are released from the primary tumors forming clusters upon TGFβ family cytokine activin treatment, which may be an initial step for malignant progression of colon cancer. (Wang D, et al, Cancer Res, 2024)
【Neutral evolution in human colon cancer metastsis】
We established single cell-derived subclones from human metastatic colon cancer, and transplanted them into the mouse spleen to examine liver metastasis. We then found that individual subclones were randomly selected and expanded to become dominant in the metastatic lesion. (Lei A, et al, J Biochem 2024)
【Cancer progression by Kras/p53 mutation and TGFβ】
We examined the gain-of-function (GOF) mechanism of missense mutant p53 using mouse and human colon cancer organoids, and found that GOF mutant p53 induces COX-2 expression, which further activates canonical Wnt signaling in neighboring cancer cells (Nakayama D, et al, Cancer Res Commun, 2025)
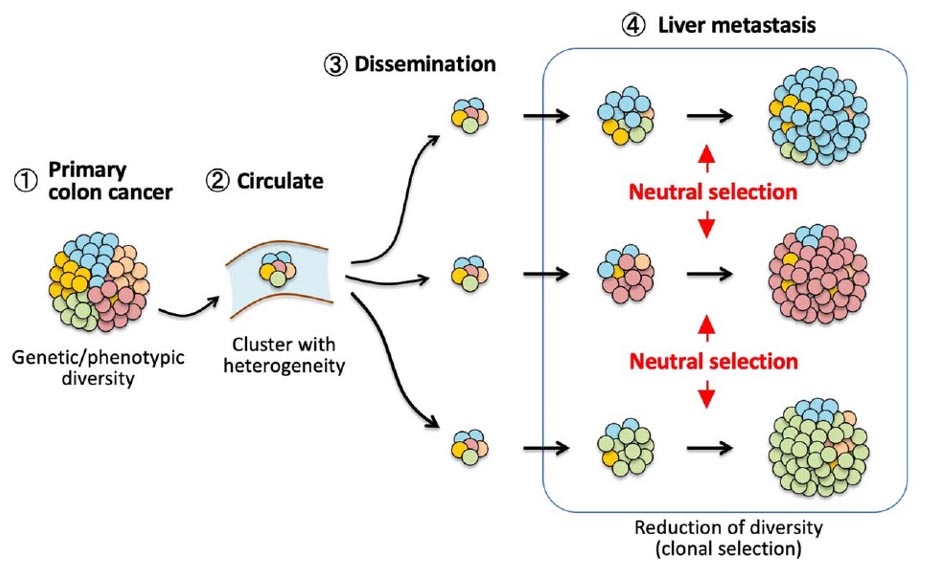
Tumor cell clusters were released from the primary colon cancer, migrated in the blood stream, and disseminated to the liver with keeping clonal heterogeneity. During expansion of disseminated cell clusters, subclones were randomly selected, and expanded to form metastatic tumors with significantly reduced diversity.
(Lei X, et al, J Biochem, 2024)
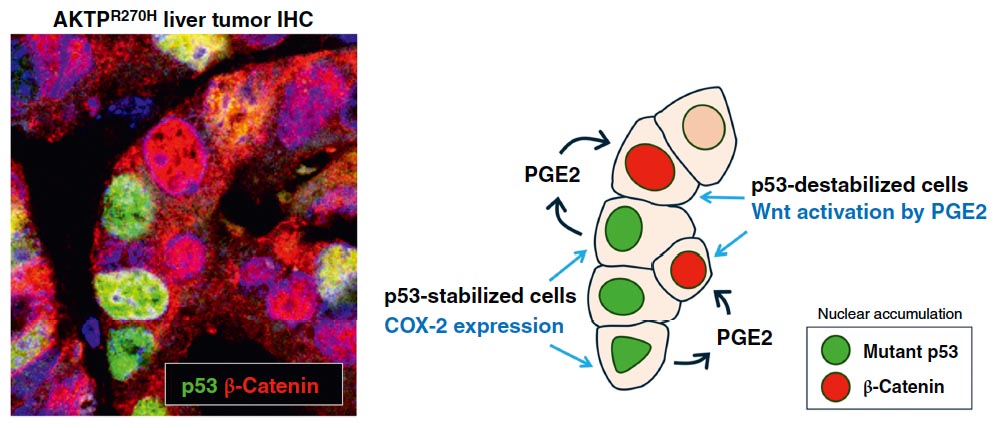
Missense mutant p53 is stabilized in cancer cells, resulting in upregulation of COX-2/PGE pathway. Secreted PGE2 then stimulates neighboring cancer cells to activates Wnt signaling pathway, which increases proliferation of cancer cells.
(Nakayama M, et al, Cancer Res Commun, 2025)

