Organization
Division of Oncology and Molecular Biology
Staff

Professor
TAKAHASHI, Chiaki

Assistant Professor
Kohno, Susumu
Aims, Ongoing Projects, and Recent Achievements
We have been investigating the ways to control the malignant progression of cancers by analyzing the molecular mechanism of various phenomena that occur when the RB1 function is suppressed. As a result, we came to the idea that it would be better to consider treatment methods for cancers those RB1 is still usable and those RB1 is no longer usable.
- Almost all oncogenic signals enhance the function of cyclin-dependent kinases (CDKs) by elevating the expression of D-type cyclins (Figure 1). This consequently induces RB1 mono-phosphorylation, which triggers full phosphorylation. Synthetic CDK4/6 inhibitors block RB1 mono-phosphorylation thereby revive RB1 functions to suppress tumors. We are trying to elucidate the detailed molecular mechanism of the efficacy of CDK4/6 inhibitors and the intrinsic resistance mechanisms in consideration of the expanded application.
- RB1 deficiency in mice can induce various tumors (Figure 2). We previously reported that loss of RB1 function not only promotes cell cycle progression, but also enhances Ras oncogenic signal and remodels intracellular metabolism and tumor microenvironment to facilitate cancer cell survival. We are currently focusing on a metabolic gene called SUCLA2 that is co-deleted upon genomic aberration involving RB1. We are developing a new drug to treat advanced prostate cancer that lacks SUCLA2.
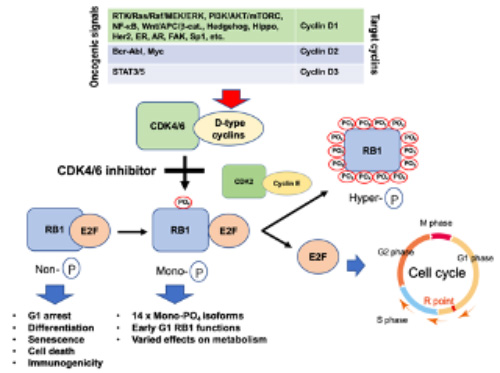
Fig.1
All oncogenic roads lead to suppress RB1 functions.
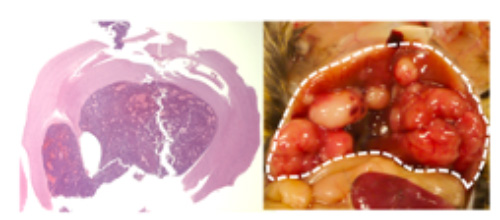
Fig.2
Brain tumor (left) and liver cancer (right) induced by inactivation of RB1 function.

