Organization
Division of Genetics
Staff

Professor
OSHIMA, Masanobu

Associate Professor
OSHIMA, Hiroko

Associate Professor
NAKAYAMA, Mizuho

Assistant Professor
WANG, Dong
Aims and Major projects
Accumulating evidence has indicated that genetic alterations as well as tumor microenvironment play a key role in cancer development and malignant progression. To examine the underlying mechanisms, we have constructed novel mouse and organoid models for gastrointestinal tumors and performed following projects.
【RNF43 mutations and Wnt-dependent colon cancer】
We established human colon cancer-derived organoids, and found that RNF43 mutant organoids proliferated in a Wnt ligand dependent manner. We further identified that treatment of PDX model with Wnt ligand inhibitor significantly suppressed tumorigenesis of RNF43 mutant cells, which may contribute to therapeutic strategy. (Yamamoto D, et al, J Pathol, 2022)
【Cancer evolution by negative selection mechanism】
AKTP organoids carrying four driver mutations in Apc, Kras, Tgfbr2 and Trp53 were established from mouse metastatic intestinal tumors. We found that about 30% of AKTP cells lost metastatic ability and eliminated from the tumor population by negative selection. (Morita A, et al, Cancer Sci, 2023)
【Cancer progression by Kras/p53 mutation and TGFβ】
Using mouse intestinal tumor-derived organoids, we found that organoids carrying Kras G12D and p53 R270H mutations are released from the primary tumors forming clusters upon TGFβ family cytokine activin treatment, which may be an initial step for malignant progression of colon cancer. (Wang D, et al, Cancer Res, 2024)
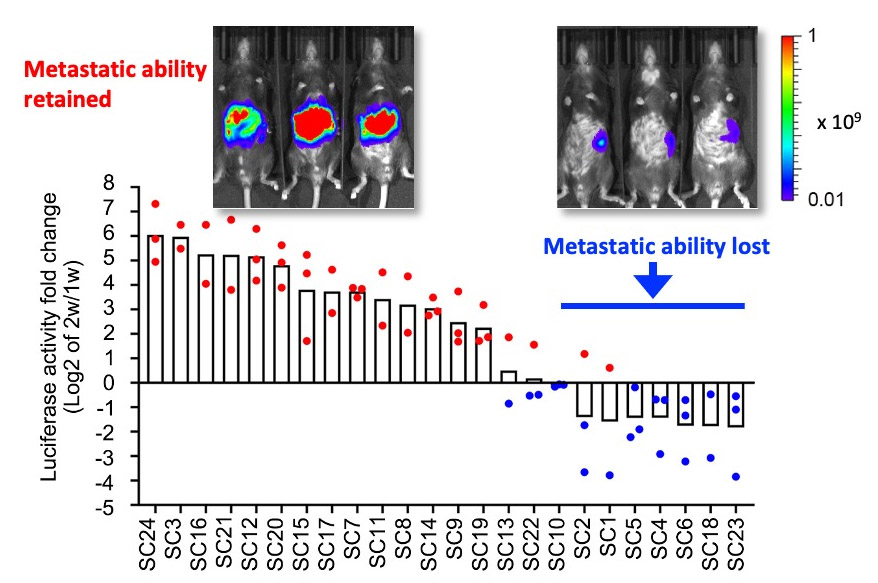
In vivo imaging analysis indicated that approximately 30% of subclones of metastatic AKTP tumor cells lose metastatic ability. Such tumor cells are continuously eliminated by negative selection from the tumor tissues. (modified from Morita A, et al, Cancer Sci, 2023)
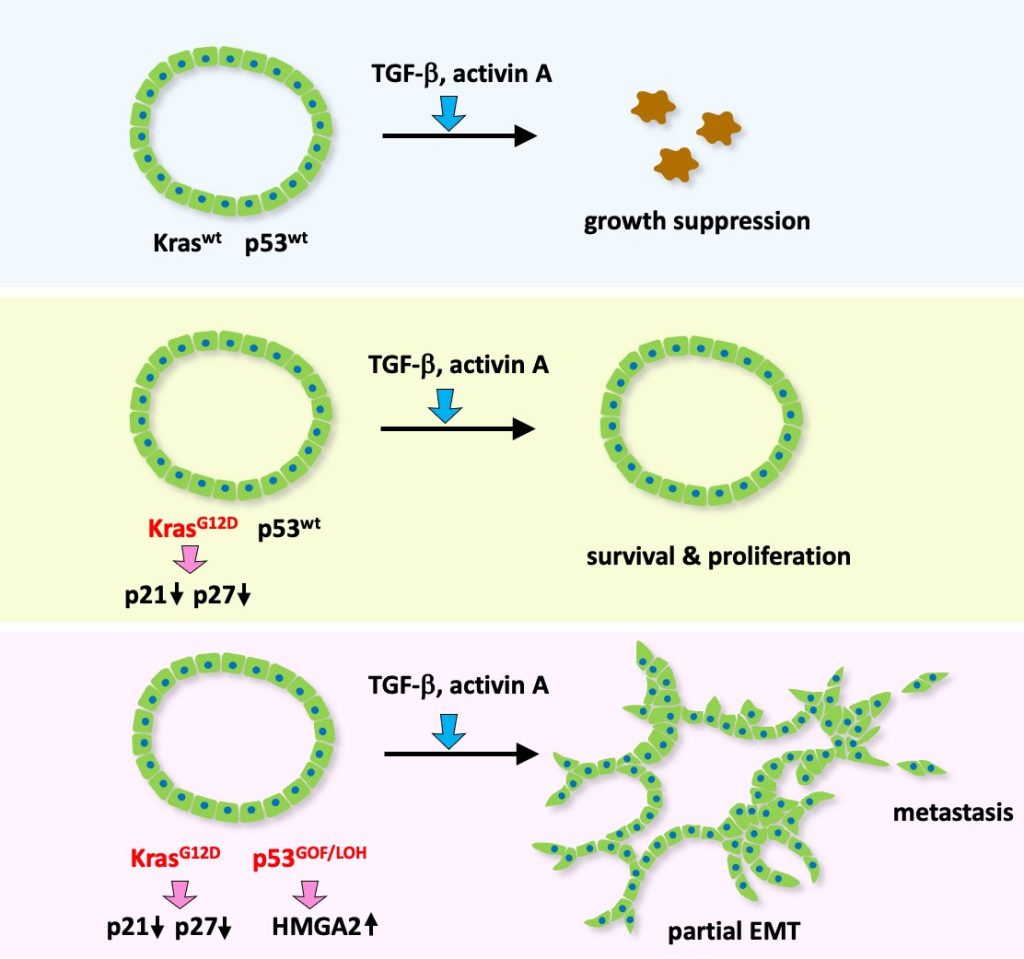
Kras/p53 wild-type tumor cells show growth suppression upon TGF/activin stimulation (top), which is suppressed by Kras mutation (middle). Additional p53 mutation causes partial EMT and metastasis upon TGF/activin signaling.
(Wang D, et al, Cancer Res, 2024)

