Organization
Division of Immune Environment Dynamics
Staff

Professor
Okamoto Kazuo
Aims and Major projects
The immune system protects our body by eliminating foreign substances such as pathogens as non-self. In cancer, tumor-derived mutant proteins can be recognized as non-self and become targets for attack by the immune system. However, tumors can evade the immune attacks by inducing immunosuppression in the surrounding tissues or by reducing immunogenicity. We study on the tumor-specific immune environment at the molecular level by unraveling the dynamic crosstalk among immune cells, tumor cells and various mesenchymal cells within the tumor tissues. We aim to develop the prevention and treatment strategies by controlling the immune environment of tumors focusing on the multicellular network.
【Mechanisms underlying the pathogenesis of bone metastasis】
Bone metastasis is a poor prognostic factor directly related to locomotor function, and thus the development of curative therapy is important. We have revealed that a soluble form of the bone-derived cytokine RANKL induces bone metastasis (Asano et al, Nature Metab, 2019) and have reported that a novel small molecule inhibitor against RANKL inhibits bone metastasis (Nakai et al, Bone Res, 2019). The bone marrow, the site of adult hematopoiesis, is composed of diverse cell populations including immune, bone, mesenchymal and endothelial cells. In bone metastases, however, the bone structure and bone marrow environment are drastically altered due to tumor progression. By focusing on the relationships between bone cells and immune cells, we aim to understand the immune microenvironment of bone metastases for the development of novel therapeutic strategies for bone metastasis.
【Molecular basis of T cell regulation】
We have elucidated the differentiation and maintenance mechanisms of Th17 cells that contribute to the psthogenesis of autoimmune diseases, and the mechanisms underlying T cell-mediated chronic inflammation and tissue repair (Okamoto et al, Nature, 2010; Inoue et al, Nature Immunol, 2018). We aim to elucidate the molecular basis of T cell responses, which would provide novel insights for development of efficient methods for induction of anti-tumor immune responses.
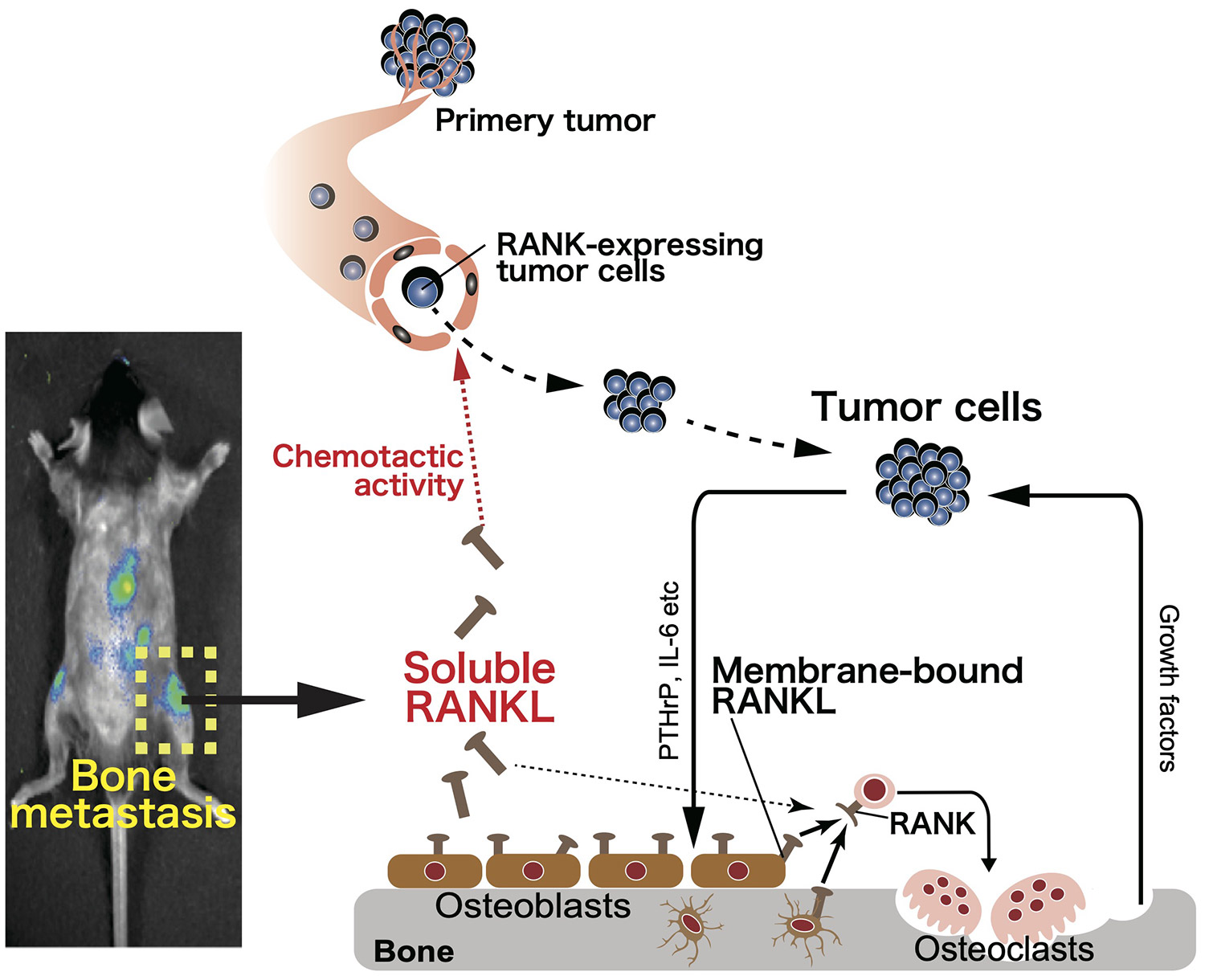
Fig.1 Soluble RANKL contributes to bone metastasis by exerting a chemotactic activity in tumor cells
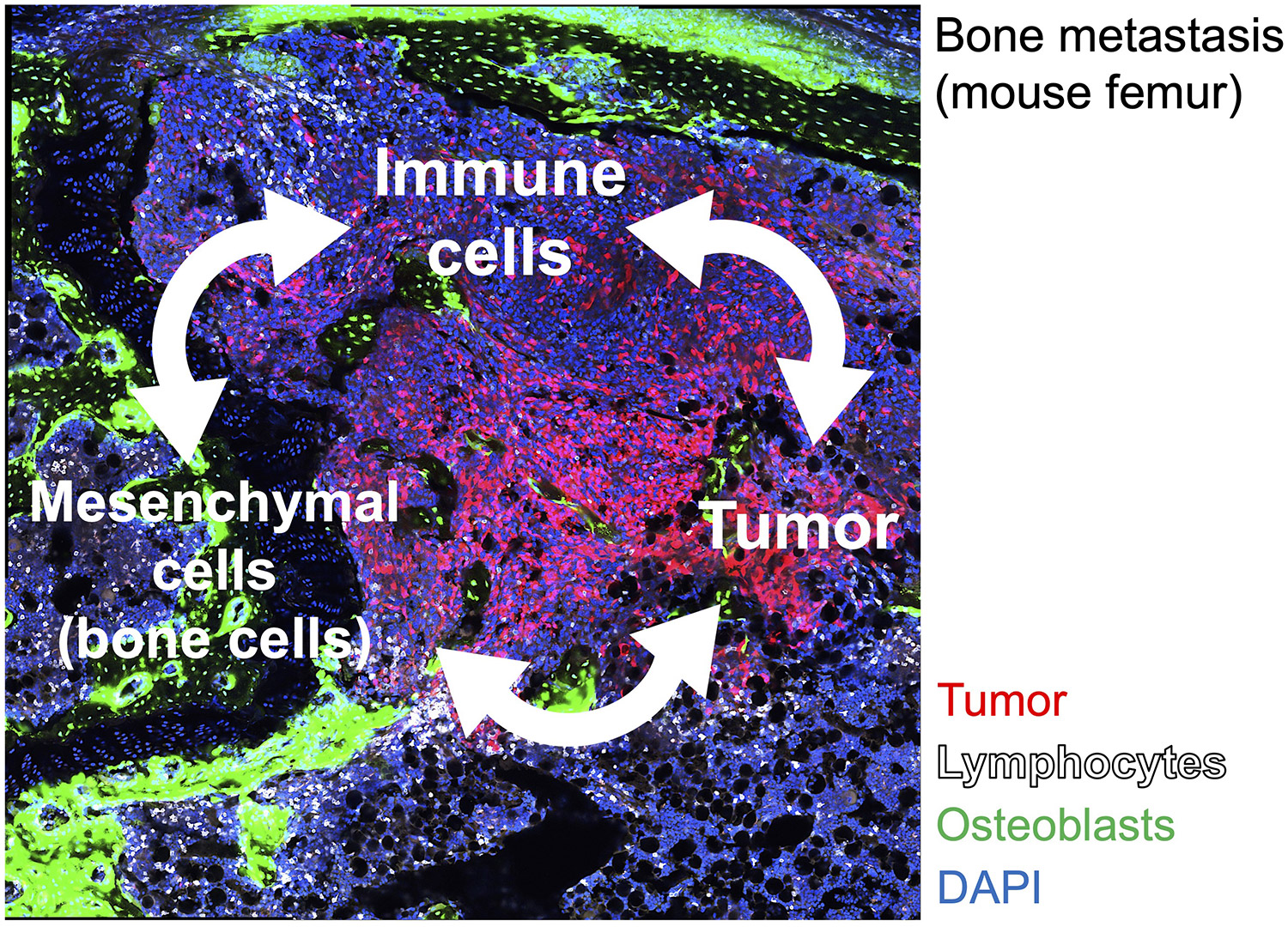
Fig.2 Understanding the tumor microenvironment consisting of cancer, immune cells and mesenchymal cells (bone cells etc)

