Organization
Division of Genome Biology
Staff

Professor
ISOZAKI, Hideko

Associate Professor
SAKAI, Katsuya

Assistant Professor
Yilmaz Neval
Aims, Ongoing Projects, and Recent Achievements
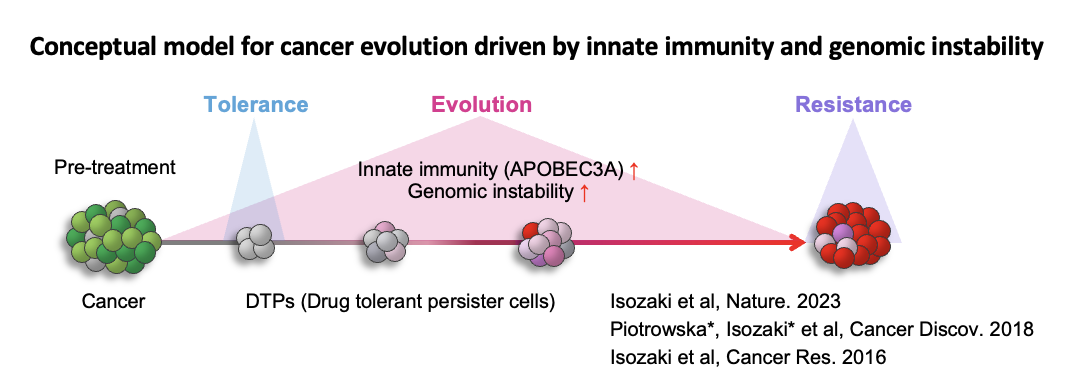
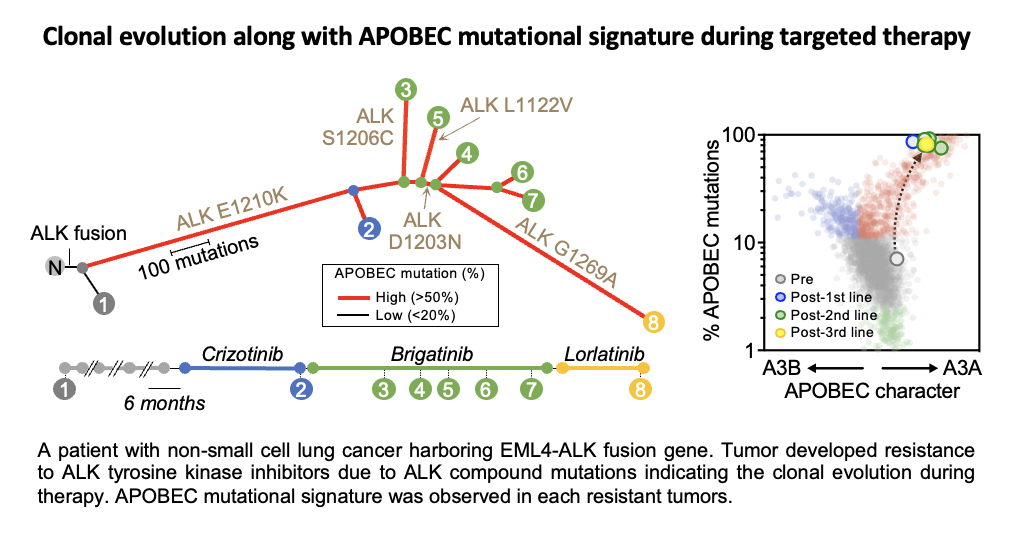
Genomic aberrations are key drivers of cancer development and drug resistance. The accumulation of somatic mutations in a single cell’s nuclear DNA, caused by external factors such as ultraviolet radiation, smoking, drugs, viral infections, and aging, ultimately leads to carcinogenesis and resistance to treatment. This process, known as clonal evolution, reflects the increasing complexity of cancer genomes over time. Although the mechanisms behind somatic mutation induction are not yet fully understood, several contributing factors have been identified. One such factor is APOBEC, a cytidine deaminase enzyme. APOBEC deaminates cytidine at the TpC motif in DNA and RNA, converting it into uridine. In our recent research, we discovered that molecular-targeted therapies induce APOBEC3A. Therapy-induced APOBEC3A increases genomic instability by generating somatic mutations and chromosomal abnormalities, which, in turn, drive the evolution of drug-resistant clones, leading to treatment failure. These findings suggest that inhibiting APOBEC3A could be a promising therapeutic strategy to prevent acquired resistance (Isozaki et al., Nature 2023). Following this report, the development of novel therapies targeting APOBEC3A is highly anticipated (Villanueva, Nat Rev Drug Discov. 2023).
Isozaki lab develops APOBEC3A inhibitors and elucidates more detailed mechanisms of clonal evolution to establish new therapeutic strategies that prevent carcinogenesis and drug resistance. In addition, we aim to open up new cancer treatments by developing therapies using genome editing technology.
1. Establishment of patient-derived cancer models
Patient derived xenograft mouse model (PDX), patient derived cell line model (PDC), organoid
2. Elucidating the mechanism of cancer evolution to develop prevention therapies
Development of nucleic acid medicine targeting APOBEC3A
Elucidation of APOBEC3A regulatory mechanisms and search for biomarkers
Comprehensive study for mechanisms of cancer evolution
3. Development of new therapy using gene editing technology
Screening for anti-cancer therapy using prime editors

