Organization
Division of Tumor Cell Biology and Bioimaging
Staff

Professor
HIRATA, Eishu

Assistant Professor
ISHIBASHI, Kojiro

Assistant Professor
LEE WAI TIK
Aims, Ongoing Projects, and Recent Achievements
Since the discovery of an oncogene encoding a protein kinase, inhibiting its activity has been considered a powerful weapon against various types of malignancy. In practice, selective inhibitors of Abl, EGFR, BRAF, etc. have shown promising clinical results. However, the emergence of resistance to these targeted therapeutics has become one of the major challenges in oncology. Our research has shown that fibroblasts present in the melanoma microenvironment play a critical role in creating a temporary drug-resistant ‘safe haven’ for BRAF inhibitors. In addition, we have found that melanoma cells in different organs respond differently to BRAF inhibitors, both clinically and experimentally. These findings clearly demonstrate the need to consider the impact of the tumour microenvironment in cancer treatment.
Our laboratory envisions a combination of a therapeutic strategy targeting the organ-specific tumour microenvironment with a medical approach based on cancer genomics as “next generation precision medicine” and aims to understand the mechanisms underlying cancer cell modification and treatment resistance by the tumor microenvironment. Currently, we are particularly focused on investigating the interaction between cancer cells and stromal cells in the central nervous system and its role in cancer progression, treatment resistance and reconstitution of the neuroimmune system. Our goal is to develop innovative treatment strategies for surgically incurable primary and metastatic brain tumours.
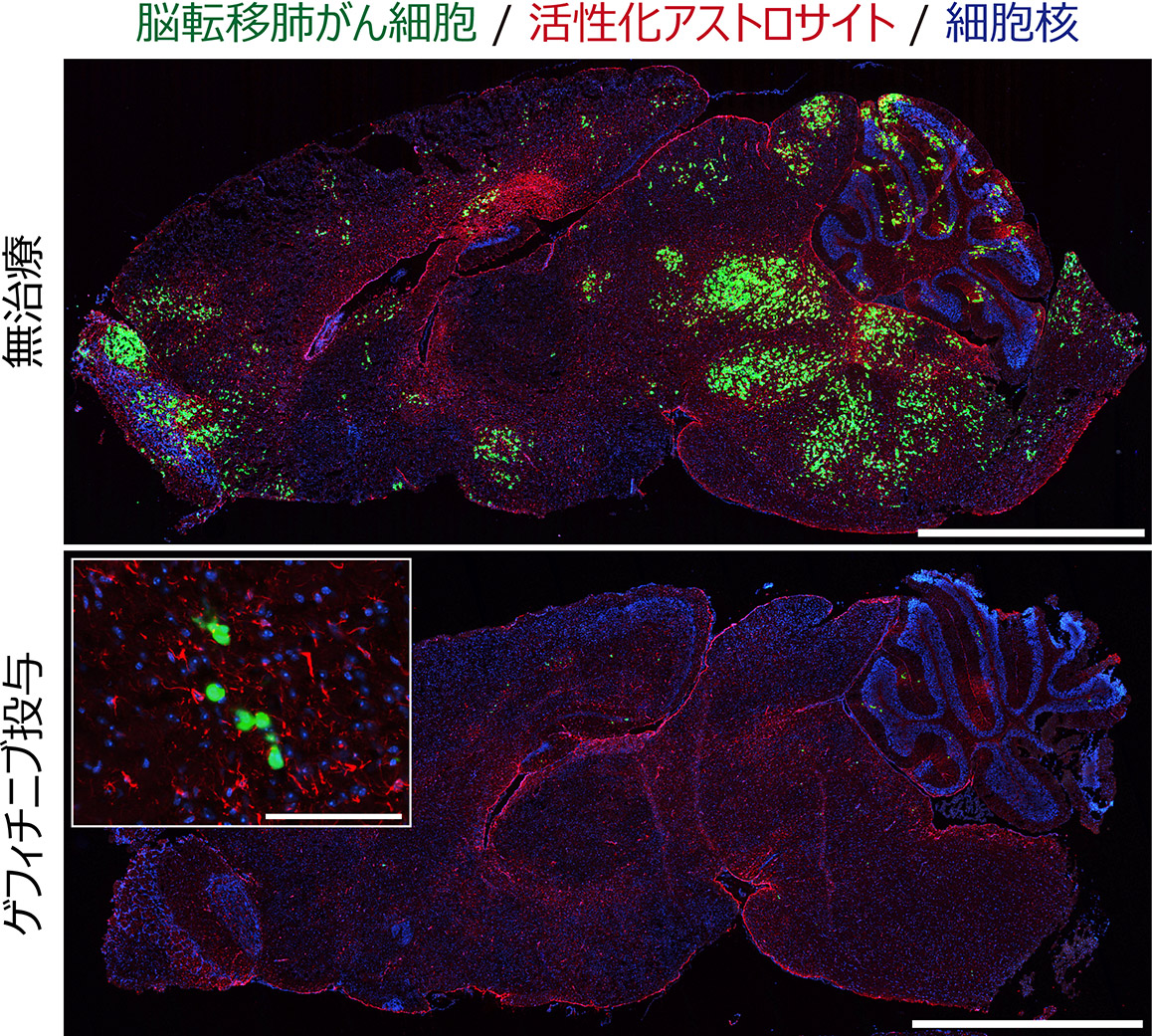
Fig.1 Drug response and resistance of brain metastatic lung cancer cells.
Brain metastatic lung cancer cells with EGFR mutations respond well to an EGFR inhibitor, gefitinib. However, the cancer cells are not completely killed and form minimal residual disease (MRD), which can act as a reservoir for relapse.
Scale = 2.5 mm (large panel), 100 mm (small panel)

