Organization
Division of Epithelial Stem Cell Biology
Staff

Visiting Professor
Nicholas Barker

Assistant Professor
MURAKAMI, Kazuhiro
Aims and Major projects
We aim to elucidate the mechanisms of self-renewal regulation of epithelial stem cells and cancer stem cells through the generation of the novel in vivo cell lineage tracing system as well as the organoids culture method. Based on these studies, we would like to proceed the regenerative medicine utilizing the capacity of tissue stem cells, and drug development for cancer prevention and therapy.
【Designation of novel human gastric tissue stem cells and cancer stem cells】
The identity of the human stomach stem cell population had been unknown. We functionally validated, the membrane protein AQP5 as a marker that enriches mouse and human adult pyloric stem cells. Furthermore, we showed stem cells within the AQP5+ compartment are a source of WNT-driven, invasive gastric cancer in vivo (Tan SH et al., Nature, 2020).
【Identification of novel genes that determine the stemness of gastric tissue stem cells】
The molecular mechanisms underlying the stemness of gastric tissue stem cells have remained a mystery. By using organoids that mimic tissue structure and function in vivo and GeCKO screening to inactivate arbitrary genes, Alk, Bclaf3 and Prkra have been identified as genes regulating stemness (Murakami K, Barker N et al., PNAS, 2021).
【Discovery of gastric cancer stem cells through analysis of a novel gastric cancer mouse model】
A lack of suitable mouse models has hampered the development of efficient therapy for gastric cancer. We developed a new gastric cancer mouse model and found novel gastric cancer stem cells that are essential for the development and malignancy of gastric tumors (Fatehullah A, Terakado Y et al., Nat Cell Biol., 2021).
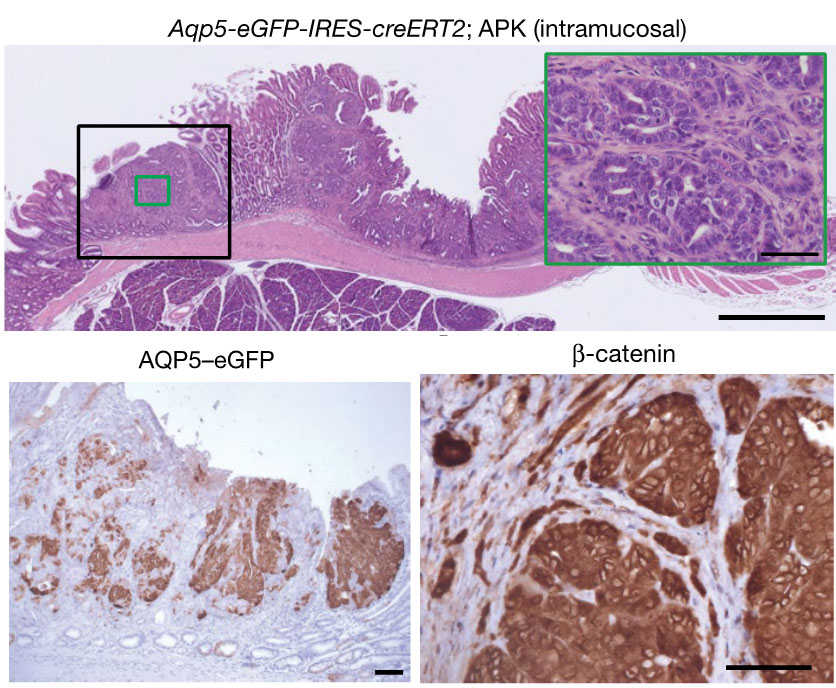
We clarified that tissue stem cells are deeply involved in the development of early gastric cancer caused by activation of the Wnt signaling pathway. Furthermore, it was revealed that AQP5-positive tissue stem cells with accumulated mutations behave as gastric cancer stem cells.
(modified from Tan SH et al., Nature, 2020)
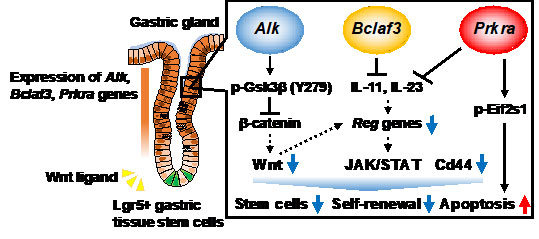
Alk destabilizes Gsk3β by phosphorylation, thus suppressing Wnt signaling. On the other hand, Bclaf3 and Prkra negatively regulate Reg gene expressions that are essential for proliferation of gastric epithelial cells through suppressing the expression of interleukins 11 (IL-11) and 23 (IL-23).
(modified from Murakami K et al., PNAS, 2021)

